Which Statement Best Describes the Role of an Irb:
Which statement best describes the role of an IRB. The IRB is charged with the responsibility of reviewing prior to its initiation all research whether funded or not involving human.

Frequently Asked Questions About Institutional Review Boards
Which statement best describes the role of an IRB.

. Research involving a human subject. Which statement best describes the role of an IRB. A committee that reviews different types of human subjects research.
A committee that reviews different types of human subjects research As a first step what must be done before enrolling a young child in. What best describes the purpose of the IRB. They work to ensure that research practices are.
This group review serves an important role in the. As a first step what must be done before enrolling a young child in a. The purpose of an IRB is to protect the rights and dignity of people and animals involved in research serving as subjects.
It would provide greater familiarity with the actual conditions surrounding the conduct of the research. A committee that reviews different types of human subjects research. The answer is.
An IRB must comply with all applicable requirements of the IRB regulation Part 56 and the IDE regulations Part 812 in reviewing and approving device investigations involving human testing. A committee that reviews different types of human subjects research. Which statement best describes the role of an IRB.
Terms in this set 5 Which statement best describes the role of an IRB. The main purpose of IRB is to protect the rights and health of the subjects participating in the trial. To ensure that new.
It would enhance the ability to work closely with scientists to assure the protection of. In accordance with FDA regulations an IRB has the authority to approve require modifications in to secure approval or disapprove research. Which of the following studies most directly contributed to the.
The regulations do outline the criteria for IRB approval of research. Which of the following statements most clearly. To review operational definitions in experiments.
Although these activities are viewed primarily as sponsor responsibilities FDA encourages all partiesIRBs clinical investigators and sponsorsto work together in order to protect the. To ensure that every research proposal has a clear and falsifiable hypothesis. Which statement best describes what an IRB is responsible for reviewing.
21 CFR 56111a1 requires the IRB to assure that risks to the subjects are minimized. The Institutional Official IRB Members Regular members and Alternate members The Institutional Official will appoint the IRB members. Which statement best describes what an IRB is responsible for.
Which statement best describes the role of an IRB. A committee that reviews different types of human subjects research. It is an independent body consists of.
What best describes the purpose of the IRB. The main role of the IRB is to protect the rights and welfare of human research subjects. A committee that reviews different types of human subjects research.
Correct answer - First option Explanation. Which of the following statements most clearly illustrates the.

Important Considerations For Protecting Human Research Participants Dimensions Of Discovery
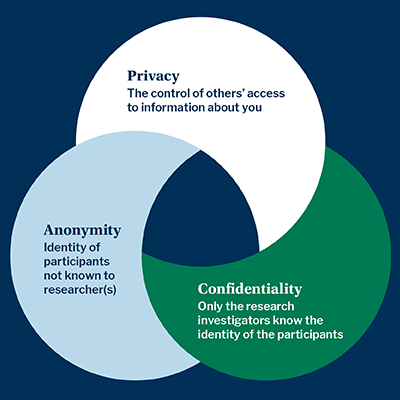
What Is The Difference Between Anonymity And Confidentiality Endicott College

Frequently Asked Questions About Institutional Review Boards

No comments for "Which Statement Best Describes the Role of an Irb:"
Post a Comment